Certifications
Full Experience of Medical Certifications
No need to worry about the problem of getting documents. With internal and external test laboratories, our experienced LAB engineers with more 10 years make sure your product certified.
Onyx can help you bring your market to USA, Europe and China quickly.
-

ISO 9001:2015
Onyx meets specifies requirements of ISO 9001:2015 for a quality management system where an organization from 2011/08/02.
Valid until: 27, February , 2025
-

ISO14001:2015
Onyx meets specifies requirements of ISO 14001:2015 for Environmental requirements, where an organization from February 2016.
Environmental Policy:
Green Manufacturing, Environmental Innovation, Sustainable ,Development Continuous ImprovementValid until: 19, February , 2022 ~ 19, February , 2025
-

ISO 13485:2016
Onyx meets specifies requirements of ISO 13485:2016 for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services from 2011/08/02.
Valid: 28, February , 2022 — 27, February , 2025
-

FCC
All of onyx's products comply with FCC Part 18.
-

CE
EN 60601-1-2: 2015 + A1: 2021 (V4.1),
EN 60601-1: 2007 + A1:2013 +A2:2021 (V3.2)
EN 55032:2015/A1:2020
EN 55035:2017/A11:2020 (ITE)
IEC 62368-1:2020+A11:2020 (ITE) -

UL : ANSI AAMI ES60601-1:2005/A1:2012/A2:2021(V3.2)
All of onyx's products have got the UL certificate.
-

cUL : CAN/CSA-C22.2 No. 60601-1:14 (IEC 60601-1: 2005+A1:2012+A2:2020,MOD) (V3.2)
-
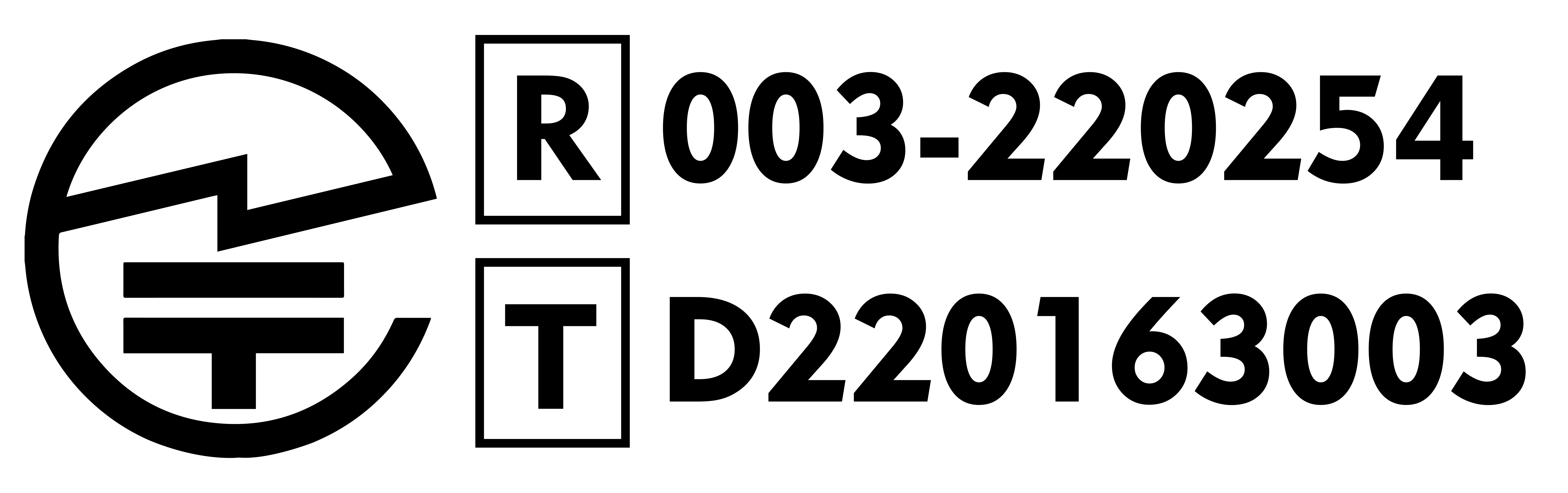
TELEC
JAPAN TELEC
-

CCC
Some of Onyx products meets the CCC mark is required for both Chinese manufactured and foreign imported products.
-

ENERGY STAR
Venus-224/244 and MATE2-2210/2410 are ENERGY STAR qualified products. Power management settings are enabled by default, allowing the display and computer to automatically enter a low-power “sleep mode” within fifteen or thirty minutes of user inactivity, respectively. The devices can be woken from sleep mode in response to a command from the power button, keyboard, mouse or the network (Wake on LAN).
-

Clorox Healthcare Compatibility Program
Onyx Products Approved Clorox Healthcare Compatible™ for Safer Medical Environments
Onyx Healthcare is the first company with EMC 4.0 / Safe 3.1 ready products
In the European Union, the Date of Withdrawal (DoW) of EN 60601-1:2006 (Safety Edition 3.0) is published as Dec. 31st 2017 and DoW of EN 60601-1-2:2007(EMC Edition 3.0) is published as Dec. 31st 2018.
All devices manufactured and imported into the EU are required to comply with EN 60601-1:2006/A1:2013 (Safety Edition 3.1) after year 2018 and EN 60601-1-2:2015 (EMC Edition 4.0) after year 2019.
All Onyx products in mass production are compliant to Safety Edition 3.1 and EMC Edition 4.0 and please refer to the following product list.

